Pharmaceutical Waste Management for Dental Practices
Dental Pharmaceutical Waste Management and the proper handling and disposal of pharmaceutical wastes has become a “hot button” issue over the last several years. Numerous studies conducted by the Federal Environmental Protection Agency, and others, have documented the presence of various pharmaceutical chemicals and their metabolic by-products in both surface and ground waters throughout the United States. As these compounds can be detected in drinking water, and as many of them have potentially serious health ramifications even at levels as low as the parts per billion range, the proper disposal of pharmaceuticals and their wastes have become increasingly important.
In 2019, the EPA finalized the Rule “Management Standards for Hazardous Waste Pharmaceuticals and Amendment to the P075 Listing for Nicotine”. The full text of the Rule can be found in the Federal Register with the publication date of February 22, 2019. The effective date of the Rule (the date by which dental practitioners must comply) was August 21, 2019. Some states may have stricter requirements in place than those the EPA has issued here. For this reason, you should always check with your state dental association to see if any such additional requirements exist in order to ensure you are in full compliance.
This Rule is extremely lengthy, complex and convoluted (covering hospitals, nursing homes, clinics, pharmacies, private practice offices of clinicians, research centers, etc.). Dental offices are specifically mentioned in the definition of healthcare facilities. In this article we will summarize all aspects of the new Rule which are relevant to dental practices including: covered materials, handling – storage – and disposal methods, record keeping, time limits, etc., so that you have a clear and concise guide to what is required of you.
The new Rule breaks down pharmaceutical wastes into various categories including P, U and D listed wastes, and further adds the headings of “non-hazardous” and “hazardous” wastes. P- listed wastes include epinephrine, nicotine (in some cases), nitroglycerine and others. U-listed wastes include, among others, chloral hydrate, while D-listed wastes include toxic materials. “Hazardous” wastes are those which possess certain properties which pose a specific risk. These include toxic agents, ignitable mixtures, corrosive agents and reactive compounds. These latter wastes, along with D- listed wastes, are not items which should be in a dental office as they are not used in the practice of dentistry. I include them here only for completeness. The other “non-hazardous” wastes – those used by dentists – are the ones we are concerned with. Remember, “non-hazardous” simply means that these wastes don’t possess any hazardous properties (like corrosiveness). These are still, however, pharmaceutical wastes and must be treated as such for disposal.
The following are parts of the Rule which are relevant to dentists:
Under no circumstances can you drain/sewer discharge pharmaceutical wastes. The purpose of the Rule is to keep these wastes out of bodies of water and waterways. It is also designed to keep these wastes out of ground water and landfill leachate.
Pharmaceutical waste may not be combined in a container with other types of medical waste (sharps and bio-hazardous). These waste streams are processed differently with pharmaceutical waste being destroyed in specially permitted facilities via high-temperature combustion.
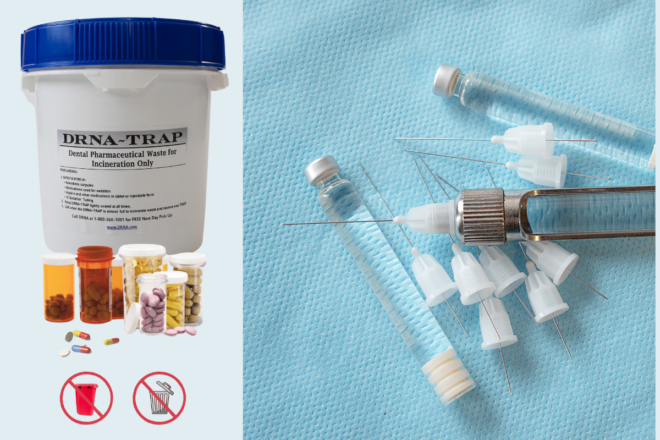
Non-hazardous pharmaceutical waste should be collected in hard-sided and leak-proof containers. These containers must be labeled as Pharmaceutical Waste. They must be blue in color (white containers with blue tops are allowed). If you were disposing of hazardous pharmaceutical waste (which you have no reason to possess in your office), or are disposing of DEA-controlled pharmaceuticals, then you would be using black containers for that purpose.
A word of caution: Any DEA controlled substances (benzodiazepines, opiates, etc.) must be segregated from the other non-hazardous pharmaceutical waste. These wastes may NOT be placed in the blue pharmaceutical waste container. These are easily disposed of by using a pharmaceutical reverse distributor. It is best to, in the opinion of this author, only stock these controlled substances in an amount which your practice will use prior to the drug’s expiration date. In this way, you can avoid having to dispose of these. When your practice purchases controlled substances, the DEA license number of the registered dentist making the purchase is accountable to the DEA. This means that you’ll need to maintain proper documentation that any of these medications, not used up through direct patient care, were properly recovered and destroyed if expired or no longer needed. If you find yourself in this position, a pharmaceutical reverse distributor can provide you with the necessary paperwork to properly document the removal of these substances from your possession.
The maximum time you can store pharmaceutical waste before sending it out for processing is 1 year.
NOTE: If you generate less than 1 kg of this waste per month you are classified as a very small quantity generator (VSQG). As such, you must ship this waste out for processing annually, or sooner if the container becomes full. (Check with your individual state to ensure they don’t require more frequent disposal). Large quantity generators (LQG) – those generating more than 100kg per month – must ship the waste out every 90 days.
Pharmaceutical waste containers must be destroyed in an environmentally protective manner by high-temperature combustion. This is to be done only in an EPA permitted facility. For this reason, be sure you are working with a reputable waste management company with a deep knowledge of dental waste regulatory issues.
When you ship out pharmaceutical waste for processing, you must maintain a signed manifest for the shipment. This signed copy must be maintained for 3 years from the date of shipment.
Failure to comply with the Rule may result in a civil penalty of up to $25,000 per day, per violation, per site and criminal penalties of up to $50,000 per day, per violation, per site – or both.
The items in a dental practice which would fall under the disposal regulations in the new Rule would include, but are not limited to:
- Partially used carpules of local anesthetic (totally empty carpules may still be placed in with sharps waste. Note: If there is liquid remaining in the carpules, you may NOT express it into a drain to empty it. You must place it in the pharmaceutical waste container for proper disposal.
- Partially used or expired medications used for parenteral, IM or IV sedation.
- IV tubing and bags which contain residual liquid. (Empty IV tubing can be treated as standard bio-hazardous waste). Empty IV bags (into which medications were not introduced) can be treated as trash. If the IV bag is empty, but did contain medication, it is not considered to be pharmaceutical waste so long as it is empty. (This author would, however, treat it as bio-hazardous waste). If the IV bag contains remaining liquid which was not administered to the patient AND a medication was introduced directly into the bag – as opposed to being introduced via a downstream port – then the bag is treated as pharmaceutical waste.
- Expired contents of “emergency kits” (nitroglycerine tablets, injectable epinephrine 1:1,000, bronchodilators such as albuteral inhalers, ammonia inhalants, aspirin, injectable histamine blockers and other similar medications whether in tablet, capsule or injectable form).
- Any prescription, over-the-counter, compounded or homeopathic drugs.
- New investigational drugs.
- Dietary supplements.
- Personal protective equipment – if contaminated with pharmaceuticals.
- Clean up material from pharmaceutical spills.
- Trans-dermal nicotine patches. The Rule is somewhat unclear here so this author suggests always placing these in with pharmaceutical waste, just to be safe.
- Electronic nicotine delivery systems (electronic cigarettes and vaping pens).
- Any liquid nicotine (vials or pre-filled cartridges) packaged for retail sale or use in any electronic nicotine delivery system.
As there has been some confusion among dentists I’ve spoken with, I am including a short list of items which ARE NOT considered pharmaceutical waste and ARE NOT regulated under this new Rule. Some of those items are:
- Composites, bonding agents, sealants and resins.
- Dental amalgam (not a pharmaceutical but regulated under a different Rule regarding amalgam).
- Sharps (not a pharmaceutical but regulated under bio-hazardous waste rules).
- Totally empty syringes (discussed previously).
- Totally empty IV bags (discussed previously).
- Unit-dose packets, wrappers, cups or blister packs are not considered as pharmaceutical waste provided the pharmaceuticals have been removed from them using common practices for removing materials from the specific type of container. (In other words, the empty containers are not considered to be pharmaceutical waste).
To summarize:
- Never drain/sewer discharge pharmaceutical wastes.
- Never dispose of pharmaceutical wastes as regular trash.
- Never dispose of pharmaceutical wastes with your bio-hazardous (red bag) or sharps waste.
- Place all non-hazardous, non-controlled pharmaceutical wastes (described above) into the specially designed containers we described.
- Use a pharmaceutical reverse distributor to dispose of DEA controlled substances.
- Ship your pharmaceutical waste container annually (for VSQG’s) to be disposed of. (Check with your individual state to ensure they don’t require more frequent disposal).
- Maintain signed manifest records for at least 3 years.
- Utilize a reputable waste management company with a deep knowledge of dental waste regulatory issues to ensure that your wastes are properly managed and disposed of. A quality company will provide you with tracking, disposal receipts and even remind you annually when it is time to ship your waste, thereby freeing you of the burden of remembering, while helping to keep you fully compliant.
In the final analysis, compliance on your part will be as easy as purchasing a pharmaceutical waste container from a dental waste management company, placing the wastes we listed into that container and having those wastes properly disposed of. While this may feel like another burden on your practice, the continuing impact of these wastes on our water supply would prove to be an even bigger burden the communities in which we live.
About Dr. Frost:
Dr. Alfred Frost holds a BA (Biology/Chemistry) from Canisius College, an MS (magna cum laude) in Epidemiology/Public Health from the Roswell Park Cancer Center and a DDS (with Thesis Honors) from the SUNY at Buffalo School of Dentistry. He has completed residencies at the Eastman Dental Center, the Univ. of Rochester School of Medicine and Dentistry and the Genesee Hospital. He has also completed fellowship-level research at the Roswell Park Cancer Center. Dr. Frost was in clinical practice and acted as the managing partner of a large group practice for over 15 years. Dr. Frost has, for the past 20 years, held the position of Vice President for Clinical and Scientific Affairs, and Director of Continuing Dental Education, at both Dental Recycling North America, Inc. and Dental Recycling International, Inc. Dr. Frost writes, lectures and consults with dentists in the areas of Hazardous Waste Management, EPA, OSHA, HIPPA, Infection Control and other regulatory and management issues impacting the profession.
For more information regarding Pharmaceutical Waste Management or other Dental Waste Management issues, contact DRNA at 1-800-360-1001 or on the web at www.DRNA.com.




